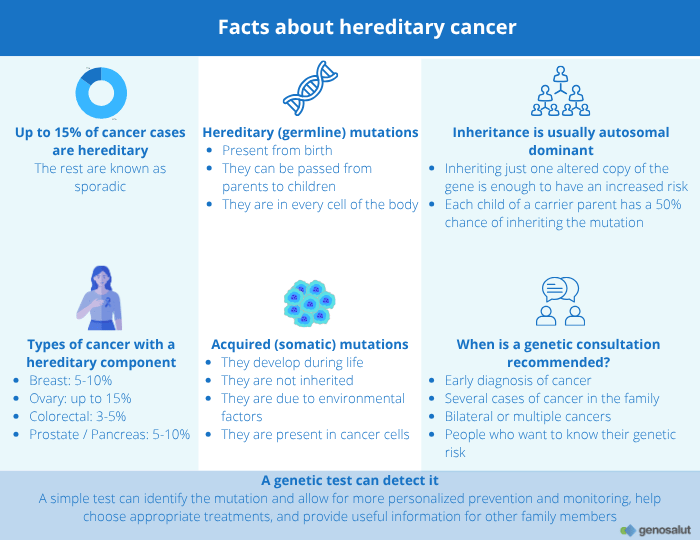
Many people wonder whether cancer can run in families. The answer is not so simple. Cancer is a complex disease that can have multiple causes. While most cases are due to environmental factors or mutations acquired throughout life, in a small percentage (between 5% and 10%) there is a hereditary genetic predisposition.
This predisposition is caused by genetic alterations present from birth that can be passed from parents to children. Understanding when cancer has a hereditary component is key to adopting preventive measures, improving early diagnosis, and offering more personalized treatment.
Cancer and genetics: how the disease develops
Cancer originates from genetic mutations that alter the normal mechanisms of cell growth and division. These mutations can cause cells to ignore control signals, divide uncontrollably, and form tumors.
There are two types of mutations involved in cancer:
– Somatic or acquired mutations: These are the most common. They occur throughout life due to environmental factors (such as smoking, radiation, infections, or diet) or spontaneous errors during cell division. They are not inherited.
– Germline or hereditary mutations: These are present from birth and can be passed down through the parents’ DNA. They affect all the cells in the body and increase the genetic predisposition to develop certain types of cancer.
The human body has multiple mechanisms to repair damaged DNA, but when these fail, errors accumulate, promoting the formation of tumors. In hereditary cancers, the pre-existing germline mutation means that the cells already start with an alteration in a key gene, reducing the number of additional changes needed for the tumor process to begin.
The difference between hereditary cancer and sporadic cancer
Although all types of cancer share the same biological origin —mutations that alter the DNA of cells— not all of these mutations appear in the same way or have the same consequences.
The vast majority of cancers are sporadic, meaning they arise over the course of life due to a combination of environmental factors, lifestyle habits, and natural cellular aging processes. In these cases, mutations are progressively acquired in specific cells of the body and are not passed on to offspring. As a result, they are usually diagnosed later in life and do not follow a clear pattern of repetition within families.
In contrast, hereditary cancers occur when a person inherits a germline mutation —that is, a genetic alteration present in all cells of the body from birth. This mutation affects key genes involved in DNA repair, cell growth control, or apoptosis (programmed cell death). Because it is inherited from one parent, it can be passed down from generation to generation, causing these cancers to appear more frequently within the same family and often at younger ages than sporadic cases.
Detecting a hereditary mutation does not mean that the person will definitely develop cancer, but it does indicate a significantly higher predisposition. Therefore, people with relevant family histories should consider undergoing genetic testing to assess their risk and, if necessary, adopt personalized monitoring or prevention strategies.
Main types of cancer with a hereditary component
As mentioned earlier, in these cases, the person is born with a germline mutation that increases their predisposition to develop certain types of cancer. These mutations are passed down through family DNA, meaning they can affect several members of the same family and recur across multiple generations.
Below are the types of cancer most commonly associated with hereditary mutations:
Hereditary colorectal cancer
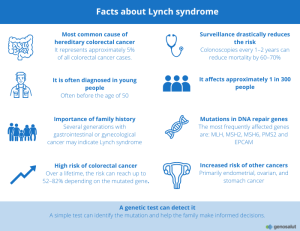
Between 3% and 5% of colorectal cancers have a hereditary origin —that is, they are caused by a germline mutation that increases the risk of developing tumors in the colon or rectum.
The most common is Lynch syndrome, but there are other hereditary forms that should also be taken into consideration.
Lynch syndrome
Caused by mutations in the MLH1, MSH2, MSH6, PMS2, or EPCAM genes, Lynch syndrome is the most common form of hereditary colorectal cancer. It increases the risk of developing colon and rectal cancer, as well as endometrial, ovarian, stomach, and urinary tract cancers.
Learn more about Lynch syndrome in this article.
Other hereditary forms
Not all hereditary colorectal cancers are Lynch syndrome. Other causes include familial adenomatous polyposis (FAP), caused by mutations in the APC gene, and MUTYH-associated polyposis (MAP), which follows an autosomal recessive pattern.
In addition, less common mutations have been identified in genes such as POLE, POLD1, NTHL1, or MSH3, which are involved in DNA repair or replication.
Hereditary breast, ovarian, and endometrial cancer (BRCA genes and others)
Between 5% and 10% of breast cancer cases and up to 15% of ovarian cancer cases have a hereditary origin, caused by mutations in genes that increase cancer predisposition.
The most well-known are BRCA1 and BRCA2, which are responsible for DNA repair. When these genes are altered, cells accumulate genetic damage, increasing the risk of developing tumors. People carrying these mutations have a higher risk of breast and ovarian cancer, in both women and men, as well as an increased risk of prostate, pancreatic, and, in some cases, melanoma cancers.
However, not all hereditary breast, ovarian, or endometrial cancers are due to BRCA. Other genes involved include PALB2, RAD51C, RAD51D, BRIP1, CHEK2, ATM, TP53, PTEN, and the MMR genes (MLH1, MSH2, MSH6, PMS2, EPCAM), which are associated with Lynch syndrome, a condition that can also cause endometrial and ovarian cancer.
Prostate and pancreatic cancer
Although the majority of prostate and pancreatic cancer cases are sporadic, between 5% and 10% may have a hereditary origin.
In many of these cases, the responsible mutations affect genes that are also associated with other types of cancer, such as BRCA1 and BRCA2, known for their role in breast and ovarian cancer. Mutations in BRCA2 confer the highest hereditary risk of prostate cancer, especially in men who develop the disease before the age of 60 or who have direct family history. Mutations have also been identified in ATM, CHEK2, HOXB13, and PALB2, which increase susceptibility.
In the case of pancreatic cancer, mutations in the BRCA1, BRCA2, PALB2, CDKN2A, and STK11 genes are involved in a significant proportion of familial cases.
Lynch syndrome may also increase the risk of this type of tumor.
Thyroid cancer and other endocrine tumors
Thyroid cancer can be part of various hereditary genetic syndromes, especially when it appears at an early age or in several members of the same family.
One of the best known is the multiple endocrine neoplasia type 2 syndrome (MEN2), caused by mutations in the RET gene. This syndrome predisposes individuals to the development of medullary thyroid carcinoma, as well as pheochromocytomas and, in some cases, parathyroid tumors. The detection of a RET mutation allows for preventive action, even leading to the recommendation of prophylactic thyroidectomy in carrier family members before the cancer appears.
On the other hand, mutations in the PTEN gene —responsible for Cowden syndrome— increase the risk of thyroid cancer (especially of the follicular type), as well as breast, endometrial, and kidney cancer.
There are also rarer syndromes, such as multiple endocrine neoplasia types 1 and 4 (MEN1, MEN4), caused by alterations in the MEN1 and CDKN1B genes, which predispose individuals to parathyroid, pancreatic endocrine, and pituitary gland tumors.
Multiple or early-onset cancers
When a person develops more than one type of cancer over the course of their life, or does so at unusually young ages, there may be an underlying genetic predisposition.
The most representative example is Li-Fraumeni syndrome, caused by mutations in the TP53 gene, which affects a wide spectrum of tumors: sarcomas, breast cancer, leukemias, brain tumors, and adrenocortical carcinomas, among others. In these cases, cancer often appears during childhood or adolescence and may recur across several family generations.
Other genes also associated with multiple cancers include PTEN, STK11, CDKN2A, SDHB, and SDHD, which are involved in syndromes that predispose to tumors in different organs.
How is cancer inherited?
In hereditary cancers, the risk of developing the disease is passed down through genetic mutations that can be inherited from parents to children.
These mutations do not cause cancer on their own, but they increase the predisposition for it to develop more easily over the course of a lifetime.
Autosomal dominant inheritance
The majority of hereditary cancer syndromes follow an autosomal dominant inheritance pattern.
This means that inheriting a single altered copy of the gene —from one parent— is enough to increase the risk of developing the disease. In this case, each child has a 50% chance of inheriting the mutation.
However, inheriting the mutation does not necessarily mean that the person will develop cancer. The onset of the disease also depends on environmental factors, lifestyle habits, and other genes that may increase or decrease the overall risk.
Other inheritance patterns
There are also less common forms of inheritance:
– Autosomal recessive: Two altered copies of the gene (one from each parent) are required to increase the risk of cancer. This is the case, for example, with MUTYH-associated polyposis (MAP).
– X-linked: The mutation is located on the X chromosome, which means it can affect men and women differently. This pattern is less common, but it has been described in some rare cancer predisposition syndromes.
Genes involved in hereditary cancer
The genes associated with hereditary cancer are generally grouped into three main categories according to their function within the cell:
– Tumor suppressor genes
These are responsible for controlling cell growth and repairing damaged DNA. When they stop functioning properly, cells may divide uncontrollably.
Notable examples include TP53, BRCA1, BRCA2, and APC.
– DNA repair genes
These correct natural errors that occur during DNA replication.
Mutations in these genes —such as MLH1, MSH2, MSH6, PMS2, or EPCAM— are responsible for Lynch syndrome and other hereditary cancers.
– Proto-oncogenes
Under normal conditions, these genes stimulate cell growth and division when necessary. However, when they mutate, they transform into oncogenes, which can lead to uncontrolled cell proliferation.
Examples include RET, MET, and KIT, which are involved in certain endocrine and gastric cancers.
When a person inherits an altered copy of one of these genes, their cells start with a genetic disadvantage: it only takes damage to the second copy of the gene to completely lose its protective function. This phenomenon, described by researcher Alfred Knudson in 1971, is known as the “two-hit hypothesis”, and it explains why people who carry hereditary mutations develop cancer more frequently or at younger ages.
The importance of genetic testing
Genetic testing is a fundamental tool for identifying whether a person has hereditary mutations that increase their risk of developing cancer.
Through a simple blood or saliva sample, it is possible to analyze the genes associated with hereditary cancer syndromes and detect variants that may have clinical significance.
Advances in genetic diagnosis
In recent years, advances in genetics and biotechnology have made it possible to develop multigene diagnostic panels that are increasingly comprehensive and precise, capable of analyzing dozens of genes simultaneously that are associated with hereditary cancer.
These studies help establish more effective prevention and monitoring plans, tailored to the genetic profile of each individual and family.
Who should consider genetic testing?
Genetic testing is not recommended for the entire population, but rather for people with specific characteristics that suggest a possible hereditary origin of cancer, such as:
– Cancer diagnosis at an early age, for example, breast cancer before age 40 or colorectal cancer before age 50.
– Several cases of the same type of cancer (breast, colon, ovary, endometrium, etc.) in different generations of the same family.
– Bilateral or multiple cancers, such as cancer in both breasts, both kidneys, or different tumors in the same person.
– Family members diagnosed with known hereditary syndromes (such as Lynch syndrome, BRCA, Li-Fraumeni, or familial adenomatous polyposis).
– Cancer in rare organs or at unusually young ages (for example, pancreatic or thyroid cancer before age 50).
– Individuals with a family history of cancer on both sides, paternal and maternal, or multiple relatives affected at similar ages.
– Patients with tumor results compatible with a germline mutation, such as microsatellite instability or loss of expression of DNA repair proteins in tumor studies.
– People who wish to know their genetic risk.
Advantages of genetic diagnosis
Carrying out a genetic study can provide multiple benefits for both the affected individual and their family:
– Personalized prevention: allows for the establishment of tailored monitoring strategies, such as colonoscopies, mammograms, or gynecological check-ups that are earlier and more frequent.
– Informed decision-making: helps assess preventive or reproductive options, including prophylactic surgery or preimplantation genetic diagnosis.
– Targeted treatments: some specific drugs, such as PARP inhibitors, are effective only in patients with mutations in the BRCA1 or BRCA2 genes.
Genetic counseling, before and after testing, is essential to properly interpret the results, understand their significance, and make informed decisions regarding personal and family care.
Hereditary cancer and prevention
Detecting a genetic predisposition to cancer does not mean that the disease is inevitable.
On the contrary, having this information provides a great opportunity to prevent it or detect it early, when treatments are most effective.
Prevention and monitoring strategies
The recommended measures vary depending on the type of mutation and the personal or family history, but generally include:
– Regular and personalized medical check-ups, adapted to the type of altered gene and the individual’s level of risk (such as mammograms, colonoscopies, ultrasounds, MRIs, etc.).
– A healthy lifestyle, including a balanced diet, regular physical activity, and avoiding tobacco and excessive alcohol consumption.
– Preventive or prophylactic treatments, such as chemoprevention or, in high-risk cases, preventive surgery to reduce the likelihood of developing certain tumors.
– Psychological support and family counseling, which are essential to guide the decision-making process and reduce the emotional impact of genetic test results.
Conclusion
Hereditary cancer represents a significant portion of all cancer cases, and its impact on families, as well as on prevention strategies, is considerable. Understanding the genetic basis of the disease allows for early action, improved diagnosis, and personalized treatments tailored to each person’s specific level of risk.
Medical genetics not only helps to understand the causes of cancer, but also drives a more preventive, precise, and human approach to medicine, where scientific knowledge and professional guidance work together to protect the health and well-being of individuals and their families.
At Genosalut, we support those who wish to understand and manage their genetic risk. Our team provides specialized counseling and personalized genetic testing with the goal of delivering accurate information that enables informed decisions and promotes long-term health.
Do you have a family history of cancer or questions about your genetic risk?
At Genosalut, we offer personalized counseling and genetic testing tailored to your situation.
Book your appointment here and take the first step toward informed prevention.