What is infertility?
Infertility is a multifactorial condition affecting approximately 15% of couples of reproductive age (1). It can be defined as the inability to achieve or complete a pregnancy after a period of one year of sexual intercourse without contraception. The causes are equally attributable to men and women.
As far as male infertility is concerned, it affects approximately 7% of males. It is characterised by very heterogeneous phenotypes, ranging from a complete absence of sperm in the testicles to various alterations in sperm quality.
Andrologists should be aware of the clinical importance of all basic genetic tests for male infertility and of the ongoing research and future prospects for androgenetics.
What are the causes of infertility?
There are multiple causes to explain the origin of infertility and several types of classification can be found in the literature depending on the approach to these causes.
Like other multifactorial diseases, environmental factors as well as genetic and/or epigenetic abnormalities and/or variants are involved in their occurrence, which makes it very difficult to determine their aetiology. It is currently estimated that genetic origin is the cause of about 20% of infertile males (2). However, this percentage is expected to increase in the coming years, as careful analysis of existing data together with technological advances, especially in next generation sequencing (NGS) methods, in expression arrays, in the use of transgenic mice, and in the use of transgenic mice (3), the use of transgenic mice (3), genome wide association studies (GWAS), epigenetic studies and microbiome studies, may possibly lead to determine the genetic origin of infertility cases currently classified as idiopathic (about 40%).
Although the causes of infertility are very varied, some articles classify them according to the following categories (4):
● Defective gamete production (problems that will lead to infertility can originate at any of the stages of spermatogenesis and ovogenesis).
● Obstruction of the reproductive tract
● Inflammation or immune dysfunction
● Sexual disturbances
In males, it is estimated that about half of all infertility cases are due to defective sperm production (1):
● Complete arrest of spermatogenesis
● Low sperm count
● Defective sperm motility
● Abnormal sperm morphology
● Abnormal sperm function
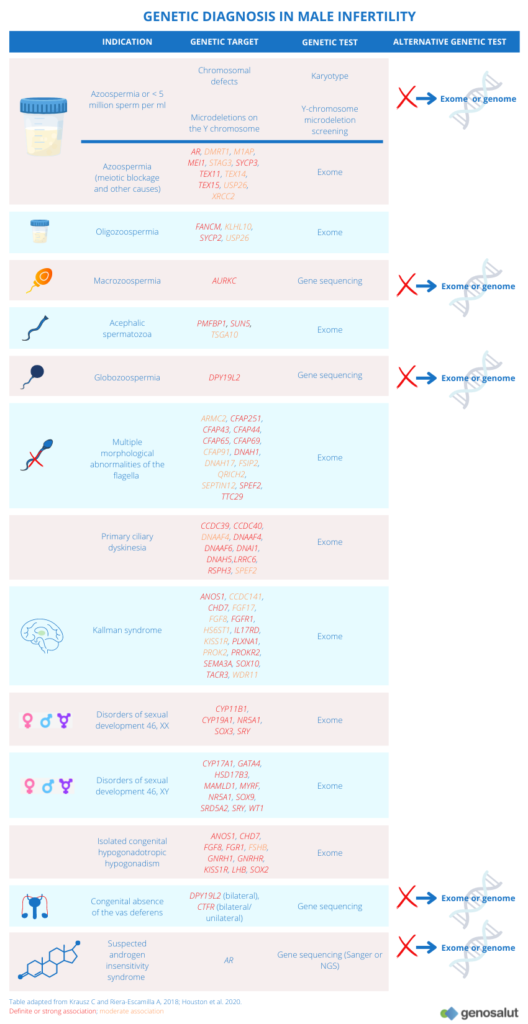
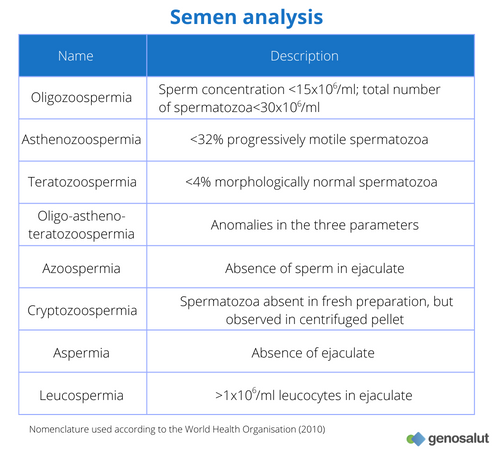
These abnormalities are related to a number of phenotypes commonly found in infertile patients (see table “Semen analysis”), and in most cases there is no information on the origin of these abnormalities. In recent decades, different research groups have focused their work on the relationship between genetic abnormalities and infertility. The best documented genetic causes of male infertility include microdeletions on the Y chromosome (5), chromosomal abnormalities (6) and CFTR gene mutations (7).
What are the genetic causes of male infertility?
The complexity of the genetics underlying male infertility is partly explained by the number of genes involved in spermatogenesis (at least 2,000) and is reflected in the heterogeneity of phenotypes observed.
Genetic factors contribute to the four main aetiological categories of male infertility:
● Quantitative spermatogenic defects.
● Ductal obstruction or dysfunction
● Hypothalamic-pituitary axis disorders
● Qualitative spermatogenic defects
The most common genetic causes of male infertility are chromosomal abnormalities such as translocations or aneuploidy of the sex chromosomes. However, other genetic defects such as deletions or mutations in the genome may be the cause of infertility.
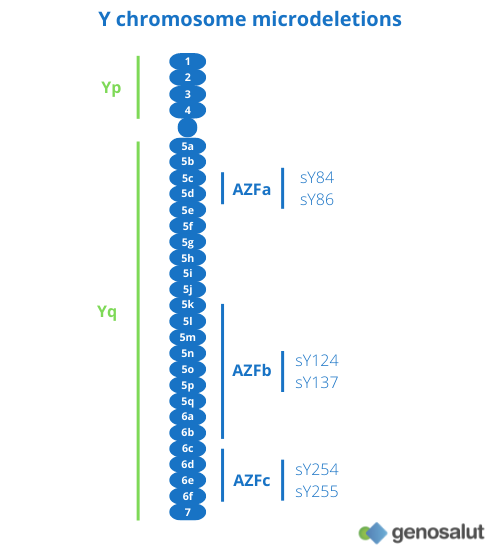
Microdeletions on the Y chromosome
The Y chromosome contains genes essential for spermatogenesis and proper development of the male gonads. Microdeletions on the Y chromosome are the leading genetic cause of infertility, with a prevalence of 10-15% in cases of non-obstructive azoospermia and 5-10% in cases of severe oligospermia (8).
There are three regions on the long arm of the Y chromosome (Yq) that have been defined as ‘spermatogenesis loci’: AZFa, AZFb and AZFc (9).
● Complete deletions in the AZFa region are associated with the absence of germ cells (10, 11).
● Deletions in the AZFb region result in blockage of spermatogenesis in primary spermatocytes (11).
● Deletions in the AZFc region have a more variable phenotype (12).
Most of these mutations are de novo and are likely to arise during meiosis of the patient’s father’s gametes. Although there are some cases of transmission of AZFc and partial AZFa and AZFb mutations, which could be attributed to oligospermic males who have achieved a natural pregnancy in the presence of a highly fertile female partner.
Chromosomal abnormalities
Chromosomal abnormalities are relatively common in humans and are among the most frequent genetic causes of infertility, recurrent miscarriages and birth of affected offspring (13-16). The frequency of somatic chromosomal abnormalities in infertile men varies from 3% in cases of mild infertility to 19% in cases of NOA (17).
In most cases, structural and numerical chromosomal alterations are thought to cause problems in chromosomal synapsis during meiosis, leading to failure of spermatogenic development. Other possible mechanisms include direct effects on genes involved in spermatogenesis (by deleting or disrupting gene sequences).
Chromosomal abnormalities are classified as numerical and structural.
Numerical abnormalities
Individuals with a 47, XYY or Klinefelter syndrome karyotype represent the most frequent aneuploidy in infertile male patients, with an incidence of 0.3%, which is 3-5 times higher than that described in the general population (18).
Patients with Klinefelter syndrome or mosaic variations of Klinefelter syndrome have compromised spermatogenesis, with severe oligozoospermia or azoospermia. The blockage of spermatogenesis usually occurs in the early stages of meiosis (19), although it has been reported that a low percentage may have sperm in the ejaculate (24).
Structural abnormalities
Structural chromosomal abnormalities can affect both the Y chromosome and the autosomes.
● Alterations of the Y chromosome include isodicentric, truncated or ring Y chromosomes. The severity of the phenotype depends on the proportion of cells with the aberrant Y chromosome (the karyotype is usually mosaic) and the degree of involvement of AZF regions.
● The most frequent structural abnormalities in the autosomes are Robertsonian translocations, reciprocal translocations and inversions.
The presence of derivative chromosomes alters the pairing and segregation of homologous chromosomes during meiosis (20-23, 25).
As a consequence, gametes carrying chromosomal abnormalities may be produced, increasing the risk of miscarriages or affected offspring. In parallel, the process of male gametogenesis may also be disrupted, leading to a reduction in the number of gametes (oligospermia), or even their total absence (azoospermia) (23).
Male 46, XX syndrome
A particular case is the 46, XX male syndrome which affects 1 in 20,000 children. Also known as De la Chapelle syndrome, it is a rare congenital intersex condition in which an individual with a 46, XX karyotype has male phenotypic characteristics that vary among those affected.
Translocation of the SRY gene (encoding a transcription factor essential for differentiation of the embryonic gonad into testes) to an X chromosome is responsible for 80-90% of cases of this syndrome. This translocation phenomenon occurs during meiosis when the two sex chromosomes (X and Y) recombine between their PAR regions. As this gene is located just below this PAR region on the short arm of the Y chromosome, it can become part of the X chromosome. When the X with the SRY gene is combined with a normal X from the mother during fertilisation, the result is an XX male.
Cases where the SRY translocation is not the causative factor (10-20%) may be caused by activation of genes downstream of SRY that are involved in the testicular determination cascade.
Most frequent genetic mutations
The most frequent mutations in individuals with fertility problems are those affecting the CFTR gene. These mutations are responsible for cystic fibrosis, and one of its manifestations is the absence of the vas deferens in the testicles. Between 60 and 90% of infertile patients with absent vas deferens carry this mutation (26). Another gene that has been associated with congenital bilateral absence of the vas deferens is ADGRG2 (27, 28).
Mutations in the AR gene, located on the X chromosome, cause a variety of defects collectively known as androgen insensitivity syndrome. Its product, the androgen receptor, plays a major role in meiotic progression and, possibly, in the formation of round spermatids (29). Mutations in AR cause azoospermia (30) and affect 2% of the infertile patient population (31).
Mutations in other genes related to other infertility phenotypes have also been identified. These include:
● Azoospermia (due to meiotic arrest):
● Globozoospermia: Mutations have been identified in four genes (DPY19L2, ZPBP, PICK1 and SPATA16). However, the prevalent (60-80%) and validated mutations are those affecting the DPY19L2 gene.
● Macrozoospermia: To date, AURKC mutations are the only validated genetic causes of sperm macrocephaly.
● Multiple morphological abnormalities of sperm flagella: this is asthenotherotherozoospermia resulting from a cluster of morphological abnormalities of sperm flagella, including absent, coiled, curled, bent, angulated, irregular or short flagella. Mutations in DNAH1 appear to be responsible for 25% of cases of this disorder. In the remaining cases the mutation could be found in one of the other 13 associated genes, which are: ARMC2, CFAP43, CFAP44, CFAP65, CFAP69, CFAP91, CFAP251, DNAH17, FSIP2, QRICH2, SEPTIN12, SPEF2 and TTC29.
● Primary ciliary dyskinesia: a rare, genetically heterogeneous, primarily respiratory disorder characterised by chronic upper and lower respiratory tract disease and asthenozoospermia due to motility defects of motile cilia and flagella. Approximately half of the patients with CPD have an organ-lateral defect. Mutations in DNAI1 and DNAH5 account for up to 30% of all cases. However, mutations in 26 other genes have been found that could lead to various ciliary structural defects and may explain 70% of the remaining cases.
● Kallman syndrome and normosomal hypogonadotropic hypogonadism: Mutations in about 30 candidate genes have been identified to date, including GNRHR, FGFR, KAL1, KISS1, TAC3 and PROK2R.
● Isolated deficiency of gonadotropins: FSH and LH.
What is the relevance of genetic diagnosis of infertility?
● Can help clarify the diagnosis.
● It can help in clinical decision making and genetic counselling. For example, it may have prognostic value for testicular sperm retrieval.
● The genetic cause of infertility is of obvious clinical importance, as it may have implications for the reproductive and general health of the patient and their offspring. Assisted reproductive techniques sometimes allow fertilisation of the egg with sperm carrying genetic defects, and therefore defining precisely those genetic defects underlying infertility is even more relevant.
● The identification of certain genetic variants is likely to help personalise hormone therapies (pharmacogenetics) in the near future.
Genetic abnormalities implicated in male infertility may also affect general health. In addition, there is a possible link between infertility and the higher morbidity and lower life expectancy observed in infertile men than in the general population.
If you like our blog, subscribe to our newsletter