What is azoospermia?
Doctors speak of azoospermia when no sperm are found in the semen. Men with azoospermia are infertile. However, those affected do not have to completely abandon their desire to become fathers. There are procedures that make pregnancy possible, such as hormone therapy or performing a testicular biopsy to extract spermatozoa that can be used for assisted reproduction.
Here is everything you need to know about azoospermia: diagnosis, causes and treatment options.
How many men suffer from azoospermia?
In terms of numbers, it is estimated to affect up to 1% of all men and between 10% and 15% of men in infertile couples.
What are the symptoms of azoospermia?
Azoospermia is usually a condition with no noticeable symptoms. Affected men usually have no complaints and it is only when the desire to have a child arises and is not fulfilled that they realise that something is wrong.
However, in some men, an underlying problem such as a chromosomal abnormality, a hormonal imbalance, enlarged testicular veins or a condition that obstructs the passage of sperm can cause certain signs and symptoms. For example, men with Klinefelter’s syndrome (90% of whom suffer from azoospermia) may have a number of general health problems such as: metabolic syndrome, autoimmune diseases, thromboembolism, cognitive or psychiatric disorders…
How is azoospermia diagnosed?
In any case, in order to diagnose azoospermia it is first necessary to perform a spermiogram or seminogram. This test allows a detailed analysis of the seminal content and determines the quality of the sperm (concentration, quantity, morphology and motility of the sperm). It is a first test when assessing male infertility, but it is by no means a definitive fertility test.
As far as sperm concentration is concerned, the values are compared with those of the World Health Organisation (WHO) reference values and a diagnosis is established:
● Azoospermia: no spermatozoa are found in the fresh sample and also not after centrifugation of the sample.
● Cryptozoospermia: no spermatozoa are found in the fresh sample but spermatozoa are found after centrifugation of the fresh sample.
● Oligospermia: the concentration of spermatozoa in the ejaculate is less than 15 million per millilitre.
● Normozoospermia: no altered semen parameters. The semen sample is within the normal range.
In order to diagnose azoospermia with certainty and to avoid analytical errors, the WHO recommends that two semen analyses be performed two to three months apart. In this way, it can be ruled out that the absence of spermatozoa in the first sample is due to factors such as stress, poor diet, taking medication, fever, etc. If in the second semen analysis the sperm count is again zero, the diagnosis of azoospermia is confirmed.
What are the types of azoospermia?
Azoospermia is usually classified into two broad groups:
● Secretory or non-obstructive azoospermia: when there are hormonal or functional problems of the testes in producing sperm (spermatogenesis).
● Obstructive azoospermia: when sperm are produced but cannot be expelled in the ejaculate due to an obstruction or absence of the ducts leading from the testicles.
In terms of causes, azoospermia has several underlying causes. Broadly speaking, it may be congenital azoospermia (i.e. it is present from birth, due to genetic or other causes) or acquired azoospermia (generally due to external factors such as toxic substances, drugs, trauma, etc.). Sometimes, it is not possible to identify the cause and we speak of idiopathic azoospermia.
Here are the main causes of non-obstructive azoospermia and obstructive azoospermia.
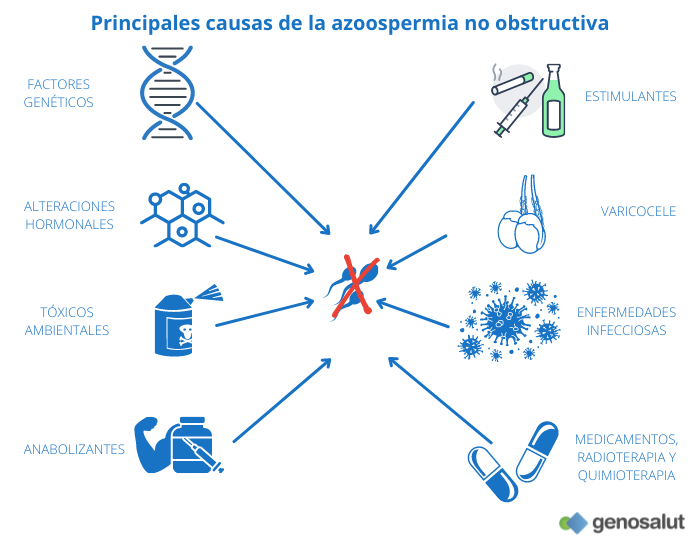
What are the main causes of secretory or non-obstructive azoospermia?
The causes of non-obstructive azoospermia are varied and range from hormonal disturbances to organ damage and genetic alterations. Triggers include:
● Genetic abnormalities: Y chromosome microdeletions, numerical chromosomal abnormalities (Klinefelter’s syndrome), structural chromosomal abnormalities (Y chromosome alterations with isodicentric, truncated or ring Y chromosomes or autosome abnormalities of the type Robertsonian translocations, reciprocal translocations and inversions), 46, XX male syndrome, mutations in certain genes (CFTR, ADGRG2, AR, TEX11…).
● Stimulants such as alcohol, cigarettes or drugs.
● Environmental toxins such as pesticides, heavy metals or solvents.
● Cancer treatments (radiotherapy and chemotherapy).
● Certain drugs, e.g. cimetidine and sulfasalazine.
● Consumption of anabolic agents.
All men who want to accentuate their muscle strength by taking anabolic steroids should be aware that these substances cause a strong decrease in the self-production of the hormones necessary for sperm production (FSH and LH), which leads to a decrease in sperm production. Even after years of abuse, the only thing that helps is hormone-boosting medication.
● Severe systemic diseases such as severe kidney or liver failure.
● Infections such as mumps virus that can cause inflammation of the testicles (orchitis). This inflammation can temporarily or permanently impair sperm development.
● Severe varicocele.
● Hormonal disorders, such as hypogonadotropic hypogonadism (deficiency of hormones released by the pituitary gland and responsible for stimulating sperm production in the testes). It may be due to a congenital disorder (Kallman’s syndrome), a pituitary tumour, or the use of anabolic steroids which suppress pituitary function.
● Congenital absence of testes (anorchia).
● Abnormal descent of the testes (cryptorchidism) during embryonic development, which are hidden in the groin or abdomen. Usually detected at birth and usually operated on at an early age. However, in bilateral cryptorchidism, most males are azoospermic despite having undergone the operation.
● Idiopathic: of unknown cause.
What are the main causes of obstructive azoospermia?
In this type of azoospermia, the testicles function properly, but the sperm cannot reach the ejaculate. Azoospermia requires bilateral obstruction of the seminal duct, either due to congenital or acquired causes (infections, operations, trauma).
● Obstruction of both vas deferens: congenital (ABCD, bilateral vas deferens agenesis) or acquired (vasectomy, bilateral inguinal hernia surgery, certain infections such as gonorrhoea).
● Obstruction of both ejaculatory ducts: congenital (Müllerian cysts) or acquired (following prostatitis).
● Obstruction of both epididymides: congenital or acquired (following epididymitis, trauma or scrotal surgery).
How is the type of azoospermia diagnosed?
Once the diagnosis of azoospermia has been established on the basis of the results of the semen analysis, there are other tests and analyses that can be used to determine the specific type of azoospermia, secretory or obstructive. These include: anamnesis, hormone analysis, physical examination, genetic study and testicular biopsy.
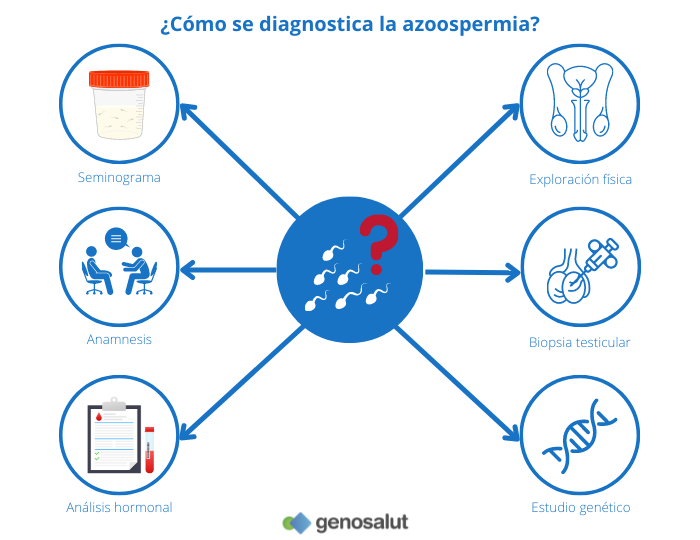
Anamnesis or medical history
Although the anamnesis is carried out at the beginning of a consultation for fertility problems, once the result of azoospermia has been obtained on the basis of the semen analysis, it can be reviewed, deepened or completed with questions aimed at this type of condition. These questions will refer to: radiotherapy or chemotherapy treatments, exposure to pesticides and toxins, taking anabolic steroids, having suffered certain diseases (mumps virus, testicular tumour, STD, varicocele, hydrocele…), testicular trauma, family history of fertility…
Hormone analysis
Sperm production is regulated by hormones, including testosterone and FSH (follicle stimulating hormone). Disturbances in the balance of the endocrine (hormonal) system can disrupt spermatogenesis and even prevent the release of sperm. Similarly, disturbances in spermatogenesis can cause hormonal imbalances.
Analysis of these two hormones can sometimes be used to determine the type of azoospermia.
● FSH: This hormone is produced in the pituitary gland (gland at the base of the brain) and its main function is to stimulate sperm production in the testicle.
Failures in spermatogenesis lead to an increase in FSH as the body’s response in an attempt to further stimulate the seminiferous tubules to restore normality. In non-obstructive azoospermia the FSH value may be elevated, in obstructive azoospermia the FSH value is usually normal, and in some pituitary diseases FSH is decreased (such as hypogonadotropic hypogonadism).
● Testosterone: This hormone, produced in the testis, follows the opposite pattern to FSH. In case of failure of spermatogenesis (azoospermia), patients have lower than usual testosterone levels.
High FSH and low testosterone values are indicative of non-obstructive or secretory azoospermia. In other words, the absence of sperm is due to a lack of sperm production, to alterations in spermatogenesis.
In contrast, normal hormonal values are indicative of obstructive azoospermia. Obstruction usually occurs at the level of the epididymis or vas deferens.
Physical examination
Physical examination includes palpation of the testicles (to look for lumps and assess their size and position), epididymis, and vas deferens. The presence of a varicocele of significant size must also be ruled out.
However, the distinction between non-obstructive and obstructive azoospermia is difficult on the basis of the above examinations. Neither physical examination (palpation of the vas deferens and measurement of testicular volume) nor laboratory tests (hormone levels) or a combination of these can establish the diagnosis with certainty.
If the results are inconclusive or if none of these additional findings are found, it is impossible to know whether it is an obstructive or non-obstructive azoospermia. A testicular biopsy provides the diagnosis.
Testicular biopsy
In men with azoospermia, a definitive diagnosis (although merely descriptive and not causative) can only be determined by testicular biopsy, which is usually performed (by testicular sperm extraction, TESE or micro-TESE) in the same surgical procedure as sperm collection for in vitro fertilisation (IVF).
The results that can be obtained are as follows:
● Mixed atrophy: tubules with different stages of spermatogenesis.
● Various types of blockage during spermatogenesis: meiotic blockage, round spermatids…
● Sertoli syndrome (SCOS, Sertoli Cell Only Syndrome) in which the tubules do not contain any germ cells.
These phenotypes can be global (present in all tubules) or focal, with a variable percentage of tubules showing various stages of quantitatively and qualitatively limited spermatogenesis.
As we have indicated, all the categories are descriptive. That is, they simply help to classify the “male factor” in the couple’s infertility, but do not offer any causal diagnosis of the alteration of spermatogenesis or the causes of obstruction in affected men.
To determine the cause of azoospermia, a valuable tool is the various genetic analyses, in particular advanced genetic studies such as exome and genome.
Classical genetic study
Genetic diagnostics in azoospermic patients has stagnated for decades in basic studies. Although they provide an answer in about 20% of cases, it is now known that the influence of genetics in this type of patient is much greater.
To date, the genetic tests indicated in cases of azoospermia are usually:
● Karyotyping: to rule out Klinefelter syndrome.
Males with Klinefelter syndrome usually have an extra X chromosome, i.e. their karyotype is 47, XXY. However, there are also cases with 2 or 3 extra X chromosomes (48, XXXY and 49, XXXXY respectively). The estimated frequency of this syndrome is 1 in 600 males in the general population, while in patients with non-obstructive azoospermia it is as high as 1 in 7. These are males with small and firm testicles, more than 90% suffer from azoospermia and the rest from cryptozoospermia or severe oligospermia.
The possibility of parenthood for these patients depends largely on two factors: age and degree of mosaicism. Assisted reproductive techniques (TESE and especially micro-TESE) allow testicular sperm retrieval in patients with Klinefelter syndrome who are azoospermic, with success rates being better in men under 31 years of age.
● Y-chromosome microdeletion screening: to rule out the presence of deletions in the AZF region.
Men may present with severe oligospermia to azoospermia depending on the location and extent of the missing fragment. However, even in cases of azoospermia, affected men can have biological children by TESE followed by ICSI. It must be borne in mind that the deletion will necessarily be passed on to the male offspring, who will therefore also have to resort to assisted fertilisation techniques to become fathers.
Since a progressive decline in sperm production has been described in men with Klinefelter syndrome and AZFc deletion, preventive sperm cryopreservation in young adulthood is a viable option for fertility preservation.
● CFTR gene mutations: cases of obstructive azoospermia are due in up to 80% of cases to the absence of vas deferens, the cause being a mutation in the CFTR gene. Virtually 100% of these patients can become fathers by removing sperm from the testicle or epididymis and using in vitro fertilisation.
However, since the frequency of CFTR mutation carriers in people of European descent is high (1 in 25), screening of the couple is mandatory to assess the risk of giving birth to a CF-affected child. If both parents are carriers, prenatal diagnosis or PGD should be performed.
As mentioned at the beginning of this section, it is already known that the influence of genetics on male infertility is much greater. In other words, with classical genetic studies, many azoospermic patients will never know the cause of their condition. In some cases, a comprehensive genetic study may be useful to find the cause of the fertility problem.
Comprehensive genetic study
Technological and scientific advances in recent years have made it possible to identify more than 100 genetic mutations associated with infertility and azoospermia. Therefore, the performance of a global genetic analysis, such as the exome or genome, which detects both the “classic” genetic factors studied to date (Klinefelter’s syndrome, microdeletions of the Y chromosome, mutations in the CTFR gene) and the new mutations detected in recent years is a tool that can contribute to the determination of the cause of azoospermia.
Apart from its direct implication for patient fertility, knowledge of the genetic causes of infertility is important for other reasons:
● Genetic variants implicated in male infertility may also affect general health.
● Genetic variants associated with infertility may have implications for the reproductive and general health of offspring.
● Improved clinical decision-making: Mutations in certain genes already have prognostic value for testicular sperm retrieval. For example, mutations in the TEX11 gene are associated with no success in sperm retrieval by TESE or micro-TESE.
● Identification of some genetic variants may help to personalise hormone therapies (pharmacogenetics) in the near future.
● Improved genetic counselling: it allows us to recommend the most suitable treatment to achieve a successful pregnancy.
At Genosalut we have more than 10 years of experience in performing and interpreting exomes and genomes for clinical diagnosis.
Is a man with azoospermia able to father a child and is it curable? A diagnosed case of azoospermia can be treated in many cases, and those affected do not have to give up the idea of having a child altogether.
What treatment is available for secretory or non-obstructive azoospermia?
● If the azoospermia is caused by a stress problem or a current drug, it sometimes helps to stop the causative agent, i.e. reduce the stress or stop taking the drug. Sometimes the testicles return to normal sperm production after a period of time.
● If the root cause is a hormonal problem, as in hypogonadotropic hypogonadism, treatment with FSH may be effective in getting the testicles to produce sperm again.
● If the problem is at the testicular level, the only alternative is to try to remove sperm from the testicle. In cases of non-obstructive azoospermia, the TESE (testicular sperm extraction) technique allows 35% of cases to obtain sperm that can be used with in vitro fertilisation techniques. The success rate of the micro-TESE technique (with surgical microscopy) can be as high as 60%. However, if no sperm can be obtained by any of these methods, the only option is to use donor sperm or adoption.
What treatment is available for obstructive azoospermia?
The reproductive prognosis in obstructive azoospermia is good. There are mainly two options:
● Surgical repair to resolve the obstruction and join the ducts (vasovasostomy, vasoepidididymostomy and transurethral resection of ejaculatory ducts).
● Sperm extraction from the testis (TESE) or epididymis (PESA), if surgical repair is not possible. The sperm retrieval success rate for IVF/ICSI is almost 100%.
Can azoospermia be prevented?
Some causes of azoospermia can be prevented. Sperm quality can be improved by a healthy lifestyle. Therefore, avoid alcohol, cigarettes, drugs and radiation.
If you have to take medication for a prolonged period of time that may affect sperm quality or undergo radiotherapy or chemotherapy treatments, there is the possibility of freezing sperm and storing it in suitable places for a later desire to have children.
Azoospermia, a condition with multiple causes that may have a solution
In brief:
● Azoospermia is a condition that affects 15% of infertile males.
● The causes of azoospermia are varied and sometimes a complete genetic study (exome or genome) can determine the cause of the problem. This is important because of the many implications, including the possibility that genetic variants implicated in male infertility may also affect the overall health of the patient and may even have implications for the reproductive and general health of offspring. In addition, some variants already have prognostic value for testicular sperm retrieval.
● The main question asked by azoospermic patients or their partners as to whether they will be able to achieve pregnancy and parenthood has no definitive answer. It will depend on the cause of the azoospermia and the possibility of obtaining spermatozoa in the testicle to be used with assisted reproduction techniques.
At Genosalut we offer both conventional genetic studies to analyse the cause of infertility and advanced genetic studies (exome).
If you like our blog, subscribe to our newsletter


